Mass Spectrometers for Interrogation of Whole Proteins
Overview
Instrumentation capable of increased characterization of intact proteins requires new approaches to design and construction. With the successful development of the separations proposed in TR&D 1, high mass proteins eluting over less than five seconds will become reality. This reality places huge demands on the instrumentation that is now required to acquire feature rich tandem mass spectra on these species. Combining mass isolation from a highly transmissive quadrupole mass filter with the speed and fragmentation efficiency of ultraviolet photodissociation (UVPD) will provide the speed required for thorough interrogation of even the most complex samples.
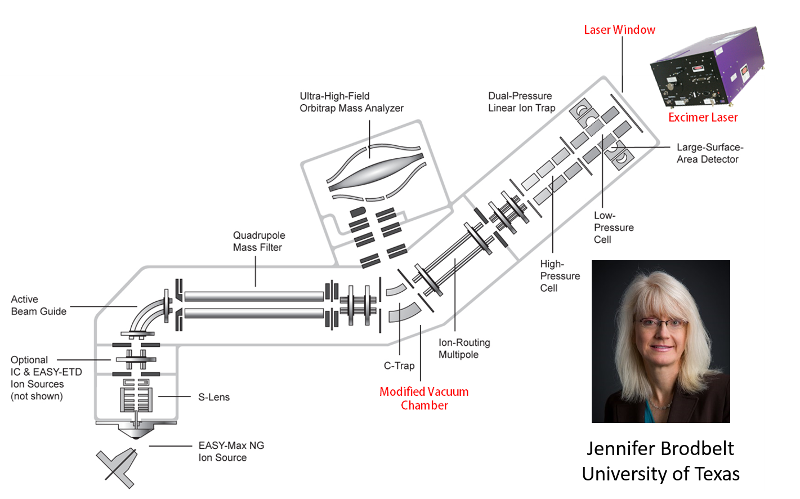
Applying Ultraviolet Photodissociation to Top Down Proteomics
High throughput top-down proteomics is a powerful tool for the identification of proteins in complex matrices. As separations and instrumentation improve, the number of protein species that may be identified in a single experiment increases. However, the most commonly used fragmentation modalities, which involve vibrational activation, generally provide incomplete fragment ion coverage. Recently, the Brodbelt lab (Satellite Center) introduced a new fragmentation method that uses brief periods (5 ns) of irradiation by a UV laser at 193 nm. UVPD provides extensive fragmentation of large proteins, enabling the nearly complete characterization of proteins even as large as 30 kDa while exhibiting far less dependence on the precursor charge state. These characteristics make UVPD an ideal addition to the already powerful toolbox of fragmentation modalities utilized in interrogating intact proteins on an LC timescale.


Comments are closed.