High Resolution Separations for Intact Proteins
Overview
To meet the needs of our driving biomedical projects, the development of intact protein separations capable of efficient, high-throughput analysis of mass-limited samples is absolutely required. To fulfill these needs, ultra-high efficiency one-dimensional separations are required to reduce sample handling, increase sample zone concentration and minimize losses. In addition, we will further refine a miniaturized version of Gel-Eluted Liquid Fraction Entrapment Electrophoresis (GELFrEE). The integrated solutions developed will increase proteome coverage by at least five-fold and accelerate the adoption of top-down methodologies by non-experts.
Slip-Flow Chromatography
Advances in top-down proteomics have been limited by the separation efficiency (i.e., the narrowness of peaks) for intact proteins. Improved efficiency is especially critical because of the multiple proteoforms of each protein and the wide range of protein concentrations. It has long been appreciated that smaller particle diameters give better separation efficiency, yet historically, particle diameters have changed very slowly.
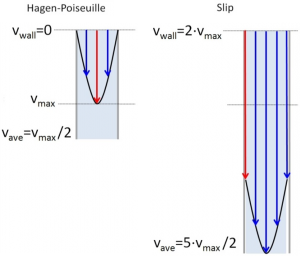
Dr. Mary Wirth, Project Lead for TR&D1 and Professor of Chemistry at Purdue University has been investigating 500 nm diameter nonporous silica particles for intact protein separations, which have a high surface-to-volume ratio. Her group has discovered that, at low particle size, a phenomenon called slip flow occurs. Slip flow gives flow rates for 500 nm particles that can approach those of 1.7 µm porous particles for the same pressure. This means that these particles can be used with existing commercial instruments.


Comments are closed.