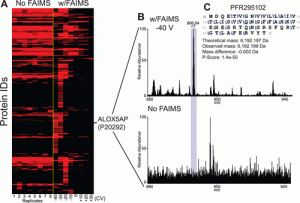
FAIMS
Description: High-field asymmetric waveform ion mobility spectrometry (FAIMS) has been implemented with differing compensation voltages (CV) values to separate proteins from mixtures and increase spectral S:N. Currently we are limited on what instruments can access the commercially available FAIMS units. The Tribrid line of Thermo mass spectrometers including the Lumos and Eclipse can adapt this technology. Studies have been performed on both mass spectrometer platforms beginning with direct reduced antibody measurements on the Lumos back in 2019. The figure below illustrates the increase in measured S:N separating our proteins with significant reduction of chemical noise. Once direct infusion studies were completed with success, targeted studies were performed with FAIMS in conjunction with LC and MS/MS fragmentation techniques. As usual top-down proteomics experiments target higher abundance proteoforms, FAIMS was useful to separate out lower abundance signals that co-eluted with other proteins. These results demonstrate the synergy and sensitivity that FAIMS has when combined with LC.
Increased sample characterization depth with FAIMS-TDMS
TR&D 5 Development Status: On going
Selected Publications:
1.Deeper Protein Identification Using Field Asymmetric Ion Mobility Spectrometry in Top-Down Proteomics Vincent R. Gerbasi, Rafael D. Melani, Susan E. Abbatiello, Michael W. Belford, Romain Huguet, John P. McGee, Dawson Dayhoff, Paul M. Thomas, and Neil L. Kelleher Analytical Chemistry 2021 93 (16), 6323-6328
2.Rafael D. Melani, Kristina Srzentić, Vincent R. Gerbasi, John P. McGee, Romain Huguet, Luca Fornelli & Neil L. Kelleher (2019) Direct measurement of light and heavy antibody chains using ion mobility and middle-down mass spectrometry, mAbs, 11:8, 1351-1357
Scientific Presentations:
ASMS 2020



Comments are closed.